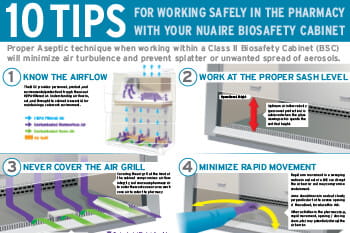
Application
Sterile Hazardous Drug Compounding
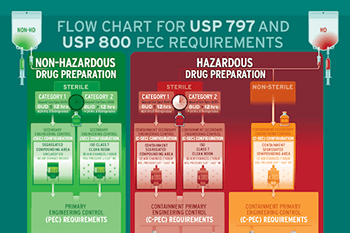
USP <800> Compliant Containment Primary Engineering Controls (C-PEC)We specialize in Containment Primary Engineering Controls (C-PEC) for sterile compounding of hazardous drugs, meeting strict USP <800> standards. Our Class II, Type A2, and Type B2 Biosafety Cabinets (BSC) are designed to improve pharmacy workflow and simplify cleaning, ensuring safety and efficacy for healthcare professionals.
Restricted Access Barrier Systems (RABS), including the Compounding Aseptic Containment Isolator (CACI), provides alternative secure hazardous drug handling with integrated glove ports. This ensures the integrity of sterile preparations and safety of both products and personnel.
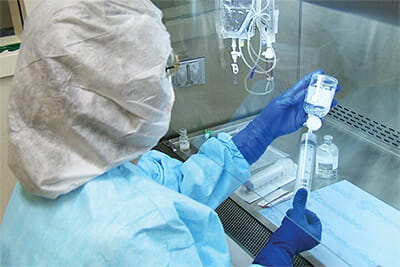
Application
Sterile Hazardous Drug Compounding
We specialize in Containment Primary Engineering Controls (C-PEC) for sterile compounding of hazardous drugs, meeting strict USP <800> standards. Our Class II, Type A2, and Type B2 Biosafety Cabinets (BSC) are designed to improve pharmacy workflow and simplify cleaning, ensuring safety and efficacy for healthcare professionals.
Restricted Access Barrier Systems (RABS), including the Compounding Aseptic Containment Isolator (CACI), provides alternative secure hazardous drug handling with integrated glove ports. This ensures the integrity of sterile preparations and safety of both products and personnel.
Our Hazardous Drug Compounding Equipment Benefits
NuAire’s sterile hazardous drug compounding equipment is designed not only to meet regulatory standards but also enhance the operational efficiency and safety in compounding pharmacy. Here’s how our equipment benefits healthcare professionals and pharmacy technicians.
Workflow and cleanability
Our containment primary engineering controls (C-PEC) are intuitively designed for workflow optimization, enabling pharmacists and technicians to work efficiently with fewer steps and less fatigue. The smooth, accessible surfaces simplify sterile environment maintenance, essential for safety and USP <800> compliance. The cleanability minimizes cross-contamination risks, improving overall pharmacy efficiency.
Ergonomics
Ergonomic design is central to our equipment development, prioritizing the physical comfort of pharmacy staff in high-pressure settings. Our biosafety cabinets and isolators feature adjustable heights and ample space, minimizing strain, reducing repetitive stress injuries, and improving focus on precise compounding tasks.
Dependability
Reliability is essential in hazardous drug compounding, and our equipment is designed for consistent performance. Built with durable, high-quality materials, our products ensure long-term dependability, minimizing downtime and maintaining steady production in high-demand pharmacy settings.
Innovative technology
We utilize cutting-edge cleanroom and containment processes to provide top-tier solutions for handling hazardous drugs. Advanced air filtration, secure barrier technologies, and automated systems ensure safe handling, protecting both the work environment and the purity of medications, which directly impacts patient safety.
Patient care and safety
Advanced compounding equipment improves patient safety by ensuring medications are prepared in a precise, controlled environment. This reduces contamination and dosing errors, resulting in better health outcomes. Enhanced workflow efficiency also allows pharmacy staff to focus more on patient care, improving overall service delivery and patient satisfaction.
Related Products

Class II, Type A2 BIosafety Cabinet
LabGard NU-543
The LabGard NU-543 pharmacy configuration features a smooth interior work zone for cleanability, exhaust airflow transition, an IV bar, and an optional base stand. Model NU-543 can be outfitted with an Ergotron arm or backwall cut out to house a monitor and keyboard for managing medication workflow systems.

Class II, Type B2 Biosafety Cabinet
Labgard nu-560
The LabGard NU-560 total exhaust biosafety cabinet is hard ducted to a facility exhaust system. A smooth interior improves cleanability for USP 800 compliance. Configure with an IV bar, base stand, and Ergotron arm to maximize compounding accuracy through aseptic technique.

Negative recirculating Compounding Aseptic Containment Isolator
pharmagard nu-nr800
The PharmaGard NU-NR800 Restricted Access Barrier System offers USP 797 and USP 800 compliance with an exhaust transition by testing to CETA CAG-002. Model NU-NR800 CACI provides an ISO Class 5 sterile work zone under negative pressure with an average of 26 air changes per minute.

Negative total exhaust Compounding Aseptic Containment Isolator
pharmagard nu-nte800
The PharmaGard Total Exhaust NU-NTE800 Restricted Access Barrier System is ducted to a facility exhaust system. Tested to CETA CAG-002 model NU-NTE800 provides USP 797 and 800 compliance by providing an ISO Class 5 sterile work zone through laminar airflow.