Article
Article
Protecting Animals and Vivarium Workers in a Rodent Facility


Most of the animals the vivarium lab personnel work with have specific pathogen free (SPF) status or origin. Moreover, many models involve immune-compromised or immune-deficient animals who can quickly become infected with microorganisms unless adequate protective measures are applied. Such infections can emanate from animal care staff/researchers, non-sterile biological products used, but also from other animals/cages. Infections can have severe effects on the health of individual animals and even can endanger the whole animal population. More often, these infections remain clinically invisible but have subtle impact on the outcome of experiments, such as a higher variability, change in the lifespan of animals, non-reproducible results, etc. Sometimes, these infections even make the animals useless for the intended experiments. For example, viral infections can occur without clinical symptoms but induce significant changes in the animals’ immune system.
Precautions to protect animal care staff/researchers from animal allergies and toxic substances, and to protect the lab animals from infection risks can be taken at different levels: 1) the level of the building, barrier systems; 2) the animal housing, cage change/transport/cleaning systems; 3) procedures level; and 4) at a personal level by the use of personal protective equipment (PPE).
This article will discuss the most frequently used types of animal housing systems and equipment for cage change and animal procedures, including how and to what level they help to protect animals and animal care staff and researchers.
Animal Housing Systems
Open Cages
Traditionally, cages with a wire-bar lid are used for housing mice and rats. Because there is a free flow of air between the inside of a cage and the room, these cages are also called “open” cages. The free flow of air, dust particles, aerosols and vapors can quickly spread from the inside of cages into the room environment. The more air movement occurs in and around the cage, the higher the chance of spreading the contents of the cage into the room environment. Risks are known to be highest during animal handling and changing and cleaning of dirty cages. If used, animal care staff and researchers can be easily exposed to animal allergens and possibly toxic research substances. Additionally, there is no animal protection against unwanted microbes from humans and other (infected) animals/cages. Therefore, infections can quickly spread between cages and are very difficult to control. Microorganisms that spread by air have caused dramatic effects on research results and continuity of animal colonies in setups with open cages and “open handling” of animals and dirty cages.
Typically, animals housed in open cages are handled open on a bench or trolly, and cage changes are performed without any protection. This causes a significant increase in dust particles in the air that transport microorganisms and allergens, especially during the stacking of dirty cages. If open cages and open” animal handling are applied, protection of animal care staff against lab animal allergens can be reached, to a certain extent, with PPE like a coverall, shoe covers, gloves and facemask. From an occupational health and safety (OHS) perspective, building construction, infrastructure, equipment and working procedures should be considered and applied first to reduce OHS risks if possible and feasible. However, PPE should only be considered the first line of defense if protection at other levels is not possible or reasonable. Despite these drawbacks, open cage systems are often used, especially in animal behavior research and toxicology settings. The method is also used in large scale SPF/SOPF breeding facilities of immunocompetent rodents in combination with stringent hygiene barriers, PPE and dedicated animal care staff.
Due to the free flow of air, open cages are neither suitable to house immune-compromised mice nor avoid potential contamination of animals with microorganisms from vivarium workers. If using an open cage, one option is to cover the cage with a closed filtertop. Typically, a Remay filter is used in these tops, preventing dust particles that can “fall” into the cage but allow some exchange of gases (oxygen, CO2, ammonia) via passive diffusion. However, the ventilation is limited, resulting in an earlier rise of higher ammonia and humidity levels. As a consequence, these filtertop cages should be changed more frequently compared to open cages.
Individual Ventilated Cages
To overcome the poor ventilation in static filtertops, a few decades ago, the Individual Ventilated Cages (IVC) concept was introduced. IVC systems are cages with closed lids and a filter at the top. Each cage has its own air supply and exhaust. The inlet and exhaust air of the system is HEPA-filtered. The result is much better air quality with significantly lower allergen levels.
Additionally, an IVC can be connected to the lab’s exhaust system, further removing allergens and odor from the room. Because the HEPA filtration of the in- and out-going air of the animal cages filter out microorganisms, the infection risk (of airborne microorganisms) is significantly reduced when cages are closed. During cage changing and procedures with animals, cages must be opened, stacked, transported, emptied and cleaned in the cage wash area only. Unless adequate protection measures are applied (both equipment and working procedures), open handling of animals and used cages will result in a temporary increase of animal and cage dust in the air, again implying higher levels of allergens and microorganisms (higher infection risk of animals/cages/personnel).
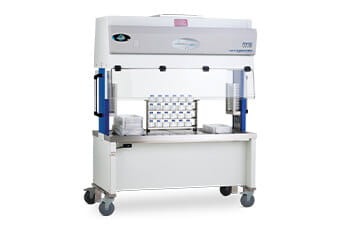
Animal Transfer Stations (ATS) & Class II Biosafety Cabinets (BSC)
Several primary engineering controls have been developed to protect vivarium technicians during the handling of animals and cages. The Animal Transfer Station (ATS) and Class II Biosafety Cabinet (BSC) are used for this purpose. Sometimes fume hoods and backdraft cabinets are also applied to protect personnel, as well as HEPA-filtered downflow modules/units. ATS’s and BSC’s protection principles are comparable: both provide a laminar (unidirectional) downflow of HEPA-filtered air in the work area where the IVC cage is opened and animals are handled to ensure product protection. However, an ATS is specifically designed for changing cages of non-infected rodents. An ATS only provides protection from most allergens.
In contrast, a Class II BSC is designed to provide a high level of personnel protection (for you and those around you), product protection (for your samples), and environmental protection (for the room). A BSC must meet Class II international standards for containment performance. To meet these high standards, a Class II BSC needs to be designed and manufactured to specific construction and performance criteria. Historically, BSC’s were primarily designed for microbiological research, and not for lab animal work. However, many BSCs described as “Animal Handling BSCs” have been modified to meet the animal researcher’s specific needs.
In contrast, when it comes to an ATS, there are no specific standards they are designed to. Each manufacturer can approach the design of their cabinet as they see fit. An ATS can be designed to be accessible from one or two sides. This provides high ease-of-use and allows for the cages/workspace to be accessed from all sides, enabling a high level of productivity (i.e., technicians doing cage changing often need to change out hundreds of cages per week). For the ease of bringing in larger cages, the sash opening height of an ATS is typically 14 inches (356 mm), whereas the maximum on a BSC generally is 12 inches (305 mm). Furthermore, an ATS has a smaller footprint, is less cumbersome, offers height adjustability, and is mobile to enable movement between IVC racks and animal rooms. This makes an ATS very versatile and widespread for general (low-risk) use.
Use of a Class II BSC is required where a higher level of protection is needed, or the animal’s status is unknown. They are used typically when working with Biosafety level 2 (BSL-2) microorganisms (wildtype as well as genetically modified) to protect the operator, the animals, and the environment. It can be advisable to use a BSC when the risk of infection of the animals should be minimized, e.g., in case of immune-deficient animals and other animal experiments that can easily be disturbed by unwanted microorganisms.
BSC’s used for animal procedural work were initially developed for in vitro procedures to protect personnel, product and environment. Later on, BSCs were redesigned and modified to facilitate working with animals and IVCs.
A BSC, by definition, is only open on one side and traditionally has an access opening height of 8 or 10-inches (203 or 254 mm). BSCs designed for animal handling need to offer a larger access opening of 12-inches (305 mm) or more to permit the movement of IVC cages in and out of the work zone. The larger access opening allows a technician to reach over the tops of cages to remove lids easily and access the interior of the cage. The added room to move arms freely above the cage allows the technician to keep arms parallel in the sterile work zone. Arms movements that involved an incline increase the risk of air traveling down the arm and out of the cabinet’s front. To ensure that Class II personnel and product protection are maintained, the user needs to be protected by a 100 FPM (0.53) air barrier through the access opening. Animal handling BSCs often feature pre-filtration systems to capture large particulates such as animal hair and dander to extend the supply and Exhaust HEPA filters’ life. BSCs are available in different sizes; 4, 5, or 6-feet (1.2, 1.6, or 1.8 m) wide. Often the room size determines what size BSC can be used.
Most BSCs used for animal work are height-adjustable and have casters for mobility for some movement and cleaning. If they are intended to be used in alternate locations, each location must be identified, and the BSC certified for proper function in each area.
Although working in an ATS and BSC may seem similar, each requires specific training. Strict standard operating procedures (SOPs) should be followed to maintain the safety level (biocontainment and bioexclusion) needed. BSCs are designed to provide a higher level of safety and are used for higher-risk situations compared to ATSs, which are intended for lower-risk applications. As a consequence, it may seem more complex to work in a BSC. However, one should keep in mind that following less strict work-procedures in an AST results in a higher risk of infections, cross-contamination, and exposure to lab animal allergens.
Laboratory animal allergen (LAA) is probably the most prominent OHS health risk in a lab animal facility. Particular attention should be paid to reduce the risk of exposing people during cage change, transport of used cages, bottles, cage enrichment and discarding of used bedding and disposables. When working in an ATS or BSC, attention to safety remains necessary. For instance, the following two routine procedures pose exposure risk for the operators unless safety precautions are brought in place: 1) when removing a cage without a lid or cover from an ATS or BSC, the downflow air is pushed into and out of the cage while moving it out of the instrument, blowing dust-containing allergens and possibly microorganisms into the breathing zone of the vivarium worker; 2) the other often neglected procedure is the stacking of dirty cages outside the ATS or BSC. Every time a cage is added to the stack, it pushes the air from the inside of the dirty cage out into the environment. The air from the dirty cages contains a high amount of dust particles with lab animal allergens and—depending on the experiment—other potentially hazardous agents. For this reason, it is recommended (and in the case of BSL or higher conditions, required) to stack cages inside the ATS or BSC. Also, care should be taken when stacks of dirty cages, used lids, tops, etc., are removed from the ATS or BSC.
To circumvent these issues by stacking of cages, a so-called “full cage change” regimen could be used. In this process, a dirty cage, lid and top are reassembled before it is removed from the AST or BSC and transported to the washing area. Compared to fully assembled IVCs (cage and closed lid), stacked cages takes far less volume per cage. As a consequence, transport and storage of stacked cages are far more efficient.
As an alternative to the full cage change, dirty cages can be stacked and bagged (as can other materials) before removing them from the AST or BSC. This is especially effective for single-use cages due to their high stacking density. Typically, a stack of 25 single-use cages/lids is 20 inches tall. A full stack of single-use clean cages or bagged dirty cages can be introduced or removed from an AST or BSC stack in a single movement. Lastly, the transport of dirty cages and other rodent housing materials should be performed in a contained way. The washing area also needs special attention as it is there that cages are unstacked, enrichment and nest material are removed, and the bedding is dumped. If no preventive measures are taken, this can cause high exposure to allergens and other hazardous agents.
Besides changing cages, a variety of procedures can be performed on animals during experiments, e.g., taking biopsies for genotyping, blood sampling, aseptic surgeries, tumor measurements, etc., including the use of anesthetic gasses or carcinogenic/toxic compounds. To protect staff from hazardous agents (including allergens), these procedures should be done inside a Class II BSC that is connected to the building exhaust system. All particles and volatile compounds will either be captured within the BSC filters or be removed from the room/building via the exhaust of the building ventilation system. For researchers who need unique experimental setups, vendors can provide custom made/designed BSCs, e.g., with integrated microscopes or heating plates to solve the demands from the research community. In certain circumstances, HEPA filter downflow module/units can be used to protect personnel and animals against hazardous substances. These units create a unidirectional downflow of HEPA filter air from top to floor or work surface. Depending on their size and construction, they can protect large surfaces for animal handling, cage changing procedures and dump stations.
Isolators
Although IVC will reduce allergen exposure and the infection risk, these cages are not 100% airtight and must be opened for cage change and animal procedures that increase contamination risk of the staff, the animals and the environment. For some type of work, more stringent barriers are necessary. In these situations, isolator systems, which can provide absolute barriers, must be used. Isolators can be made of flexible film, plexiglass, or stainless steel combined with safety glass. Both the supply and exhaust air are generally HEPA filtered. In an isolator, animals are housed in open cages. For the introduction and removal of materials (feed, water, bedding and other materials needed), a unique material sluice is used to prevent breaking the barrier. Depending on the animal study, materials must be sterilized before introduction into or after removal from the isolator as must the isolator itself. Within the wall of the isolators, long sleeves are mounted for handling the animals and cages and to perform animal procedures.
Isolators can be equipped with particular docking ports for docking to a biosafety cabinet or a second isolator so animals can be moved between an isolator and a BSC for procedures that are not feasible within an isolator or between isolators. A determination as to which safety level/level of containment instrumentation is needed depends on the samples that need to be contained, as well as the safety level of the environment in which the procedures are performed. In the case of hazardous agents, a risk assessment must be completed and identified actions are accomplished before the study is started.
Isolators either run on positive or negative pressure. Positive pressure ensures that, in case of small holes (e.g., in a gasket, the flexible film or sleeves), no microorganism enters the isolator and thereby provides optimal bio-exclusion. Positive pressure isolators are mostly used for germ-free, nucleus breeding colonies and microbiome studies. Isolators run in negative pressure ensure that even in the case of small leaks, no air or microorganisms can escape from the isolator, ensuring optimal bio-containment.
Isolators either run on positive or negative pressure. Positive pressure ensures that, in case of small holes (e.g., in a gasket, the flexible film or sleeves), no microorganism enters the isolator and thereby provides optimal bio-exclusion. Positive pressure isolators are mostly used for germ-free, nucleus breeding colonies and microbiome studies. Isolators run in negative pressure ensure that no air or microorganisms can escape from the isolator, ensuring optimal bio-containment even in the case of small leaks.
Isolators are large and bulky (poor ergonomics, low number of animals/m2 or foot2), and all animals within an isolator must have the same microbiological level. This means that for experiments with test groups with different microorganisms, a separate isolator is needed for each test group. Furthermore, if there is a microbiological break in an isolator, the complete content should be considered as contaminated and potentially lost. Working with isolators demands a certain attitude, skill and experience. Therefore, isolators often have a dedicated team of animal care staff and are not easily accessible for other staff, including researchers.
Micro-Isolator cages
With increasing interest in germ-free animals comes an increase in microbiome studies and work with infectious and hazardous material, which needs a higher protection level than standard IVCs provide. There was a clear need for more flexible, scalable systems that are accessible for researchers. Some vendors developed a specially designed IVC caging system with a higher containment level, referred to as micro-isolator sages. Such cages typically have HEPA air filtration on the individual cage level, are sufficiently airtight and can be submerged in disinfectant for external decontamination. By docking into an IVC rack, the valve for air supply and exhaust is opened. Micro-isolators should/must be opened and handled within the protective environment of a BSC Class II or higher, depending on the risk classification of the hazardous agents used and the results of a risk analysis.
As with isolators, there are two types of micro-isolators with crucial differences. One type has positive pressure for bio-exclusion studies—germ-free, Gnotobiotic animals, immune-suppressed animals, microbiome studies. The second type runs on negative pressure and can be used for animal experiments that need a high level of biocontainment, including BSL-III microorganisms.
Conclusion
Animal transfer stations and biosafety cabinets are an effective solution for vivarium workers that need protection from animal allergens, as well as instrumentation that provides good animal husbandry and care of animals. These containment instruments also minimize infection risk of personnel and positively impact study results, if used correctly. This article has provided an overview of the different types of rodent housing systems and equipment used for cage changing and procedures on animals, detailing how they protect animals, animal care staff, researchers and the overall laboratory environment.